H.A. Pérez-Ramírez, A. Moncho-Jordá, and G. Odriozola
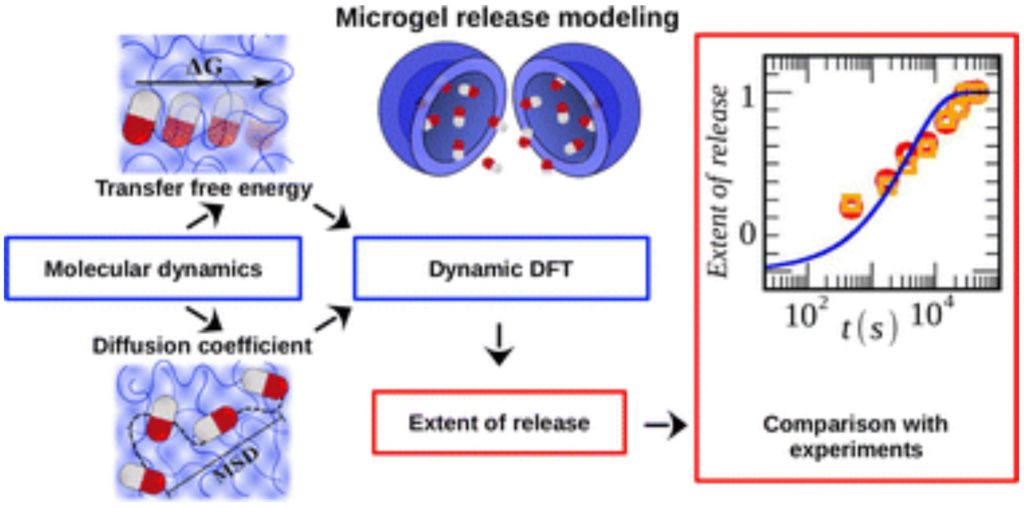
We employed molecular dynamic simulations (MD) and the Bennett’s acceptance ratio method to compute the free energy of transfer of phenol, methane, and 5-fluorouracil (5-FU), between bulk water and water–pNIPAM mixtures of different polymer volume fractions. For this purpose, we first calculate the solvation free energies in both media to obtain the free energy. Phenol and 5-FU (a medication used to treat cancer) attach to the pNIPAM surface so that they show negative values of free energy irrespective of temperature (above or below the lower critical solution temperature of pNIPAM (LCST). Conversely, methane switches the free energy sign when considering temperatures below (positive) and above (negative) the LCST. In all cases, and contrasting with some theoretical predictions, the free energy maintains a linear behavior with the pNIPAM concentration up to large polymer densities. We have also employed MD to compute the diffusion coefficient of phenol in water–pNIPAM mixtures as a function of the polymer volume fraction in the diluted limit. Both, the free energy and the diffusion coefficient are required inputs to obtain the release halftime of hollow pNIPAM microgels through Dynamic Density Functional Theory (DDFT). Our scaling strategy captures the experimental value of 2200 s for 50 mm radius microgels with no cavity, for polymer volume fractions of 0.83 at 315 K.