Luis Pérez-Mas, Alberto Martıín-Molina, Manuel Quesada-Pérez Arturo Moncho-Jordá
In this work the equilibrium absorption of nanometric cosolutes (which could represent drugs, reactants, small globular proteins and other kind of biomacromolecules) inside neutral hydrogels is studied. We specially focus on exploring, for different swelling states, the competition between the steric exclusion induced by the cross-linked polymer network constituting the hydrogel, and the solvent-induced shortrange hydrophobic attraction between the polymer chains and the cosolute particle. For this purpose, the cosolute partition coefficient is calculated by means of coarse-grained grand canonical Monte Carlo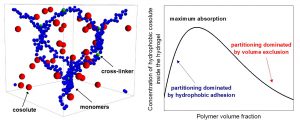 simulations, and the results are compared to theoretical predictions based on the calculation of the excluded and binding volume around the polymer chains. For small hydrophobic attractions or large cosolute sizes, the steric repulsion dominates, and the partition coefficient decreases monotonically with the polymer volume fraction, fm. However, for large enough hydrophobic attraction strength, the interplay between hydrophobic adhesion and the steric exclusion leads to a maximum in the partition coefficient at certain intermediate polymer density. Good qualitative and quantitative agreement is achieved between simulation results and theoretical predictions in the limit of small fm, pointing out the importance of geometrical aspects of the cross-linked polymer network, even for hydrogels in the swollen state. In addition, the theory is able to predict analytically the onset of the maximum formation in terms of the details of the cosolute–monomer pair interaction, in good agreement with simulations too. Finally, the effect of the many-body attractions between the cosolute and multiple polymer chains is quantified. The results clearly show that these many-body attractions play a very relevant role determining the cosolute binding, enhancing its absorption in more than one order of magnitude.
simulations, and the results are compared to theoretical predictions based on the calculation of the excluded and binding volume around the polymer chains. For small hydrophobic attractions or large cosolute sizes, the steric repulsion dominates, and the partition coefficient decreases monotonically with the polymer volume fraction, fm. However, for large enough hydrophobic attraction strength, the interplay between hydrophobic adhesion and the steric exclusion leads to a maximum in the partition coefficient at certain intermediate polymer density. Good qualitative and quantitative agreement is achieved between simulation results and theoretical predictions in the limit of small fm, pointing out the importance of geometrical aspects of the cross-linked polymer network, even for hydrogels in the swollen state. In addition, the theory is able to predict analytically the onset of the maximum formation in terms of the details of the cosolute–monomer pair interaction, in good agreement with simulations too. Finally, the effect of the many-body attractions between the cosolute and multiple polymer chains is quantified. The results clearly show that these many-body attractions play a very relevant role determining the cosolute binding, enhancing its absorption in more than one order of magnitude.
